Methylene Blue (chemical name methylthioninium chloride) was first synthesized in 1876 by German chemist Heinrich Caro . Originally developed as a textile dye (the first synthetic dye from coal tar), it soon attracted medical interest. In 1891, Paul Ehrlich and Paul Guttmann pioneered its use against malaria, successfully curing two patients with Methylene Blue . This made it the first synthetic drug used in medicine, even before antibiotics . Early clinicians believed the blue dye “stained” parasites and bacteria to kill them – a simplistic theory, but it led to real treatments . Methylene Blue was also tried as an analgesic in 1890 for nerve pain and rheumatism and as one of the first therapies for psychosis (hallucinations and delusions) in the late 19th century . In fact, its use in psychiatric patients about 1890–1900 predated modern antipsychotics by decades, and later research on Methylene Blue helped inspire the development of the phenothiazine class of antipsychotic drugs (which includes chlorpromazine) .
Early in the 20th century (before antibiotics), Methylene Blue was tried for various infections. Doctors used it as a urinary antiseptic for bladder infections (UTIs) and as a remedy for cyanide poisoning. It was even combined with other agents in pills for urinary tract pain and infection (e.g. the old “Prosed/DS” formula) . However, while lab tests showed Methylene Blue could kill bacteria, in practice these pre-antibiotic UTI treatments were largely disappointing . Soldiers in World War II took Methylene Blue for malaria, but humorously complained that “even at the loo, we see, we pee navy blue” – referring to the dye turning their urine vivid blue . Despite its quirks, Methylene Blue earned a place as a versatile remedy. By the mid-20th century it was recognized for treating methemoglobinemia, a condition where blood cannot carry oxygen. It’s now on the World Health Organization’s list of Essential Medicines for that life-saving use .

Mechanism of Action
Mitochondria as the “Powerhouse”: To understand Methylene Blue’s cellular effects, it’s important to know how mitochondria generate energy. Mitochondria are tiny organelles in our cells that produce ATP, the cell’s energy currency, through a process called oxidative phosphorylation. Inside mitochondria, an assembly line of proteins called the electron transport chain (ETC) passes electrons derived from nutrients (like glucose) along a series of complexes (I through IV) embedded in the inner mitochondrial membrane . As electrons flow down the chain (a bit like a relay race), complexes I, III, and IV pump protons (H⁺) across the membrane, creating a proton gradient. This proton flow then drives ATP synthase (Complex V) to crank out ATP – much like water turning a turbine to generate electricity. Oxygen serves as the final electron acceptor at Complex IV, where it’s reduced to water. In short, mitochondria convert fuel and oxygen into ATP, powering cellular functions. The downside is that sometimes electrons leak prematurely (especially at Complex I and III), forming reactive oxygen species (ROS) that can damage cells .
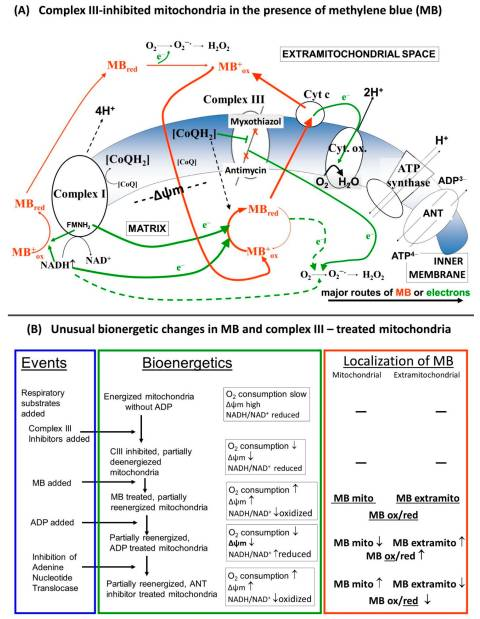
Methylene Blue’s role as an electron carrier: Methylene Blue (MB) has a unique redox ability – it can oscillate between an oxidized blue form and a reduced colorless form (called leucomethylene blue). In doing so, MB can accept and donate electrons. Inside mitochondria, low doses of Methylene Blue can act as an alternative electron carrier in the ETC. Specifically, MB is able to pick up electrons upstream (for example, from NADH via Complex I) and then donate electrons directly to downstream components like cytochrome c, effectively bypassing Complex I and Complex III . By “shuttling” electrons along, MB provides an alternate pathway for electron flow when the normal chain is impaired . This keeps the electron relay race running and helps maintain the proton gradient for ATP production. Not only can MB sustain energy production under stress, it also reduces oxidative stress: by rerouting electrons, it prevents the build-up of electron pressure that causes leakage at Complex I/III and thus produces fewer ROS . In essence, MB acts like a backup runner in a relay – if one of the key runners (Complex I or III) falters, MB can grab the electron “baton” and hand it off further down the line, ensuring the race (ATP production) still finishes. This keeps mitochondria working efficiently and cells energized even under conditions that would normally choke off their energy supply. Studies show that MB’s presence improves mitochondrial respiration and preserves the membrane potential when parts of the ETC are blocked . It significantly boosts Complex IV activity and oxygen consumption while cutting down on ROS generation .
Simple analogy: Imagine the electron transport chain as a relay race with four runners (Complex I, II, III, IV) passing a baton (electrons) to reach the finish line (oxygen, which gets converted to water). If runner #1 or #3 gets tired or drops the baton, the race would slow down and chaos (ROS sparks) might ensue. Methylene Blue steps in like an alternative runner who can snatch the baton and directly hand it to a later teammate (cytochrome c/Complex IV), skipping over the missing runners. The race continues smoothly, and the team still produces energy (ATP) without a pile-up of electrons. By acting as this relay substitute, MB helps cells keep producing energy and prevents the “spillover” of electrons that would have formed harmful free radicals . (Notably, MB doesn’t interfere with Complex II’s separate input from succinate/FADH₂, so it mainly supplements the NADH pathway). This multi-faceted mechanism – maintaining ATP output while lowering oxidative stress – underpins many of Methylene Blue’s potential benefits in health and disease.
Potential Benefits
Cognitive enhancement and brain energy: Because Methylene Blue improves mitochondrial efficiency, it can have notable effects on the brain, our most energy-demanding organ. Research suggests low-dose MB has nootropic (cognitive-enhancing) properties. In animal studies, a single small dose of MB enhanced memory retention and learning . Building on this, a placebo-controlled study in healthy humans found that a single oral dose of MB led to sharper memory and attention. Functional MRI scans showed increased activity in brain regions responsible for memory and focus, and memory test scores improved by 7% compared to placebo . Users often report feeling more clear-headed and energetic on low-dose MB, likely due to more efficient ATP production in brain cells. By promoting better cellular energy output (and possibly increasing neurotransmitter levels like serotonin and noradrenaline via MAO inhibition ), MB may support overall mental energy, alertness, and mood. Some psychiatrists have even tested MB as an adjunct for depression – one trial in severely depressed patients showed that 15 mg of MB daily for 3 weeks significantly improved symptoms versus placebo . These cognitive and mood benefits link back to MB’s mitochondria-boosting and neuroprotective actions.
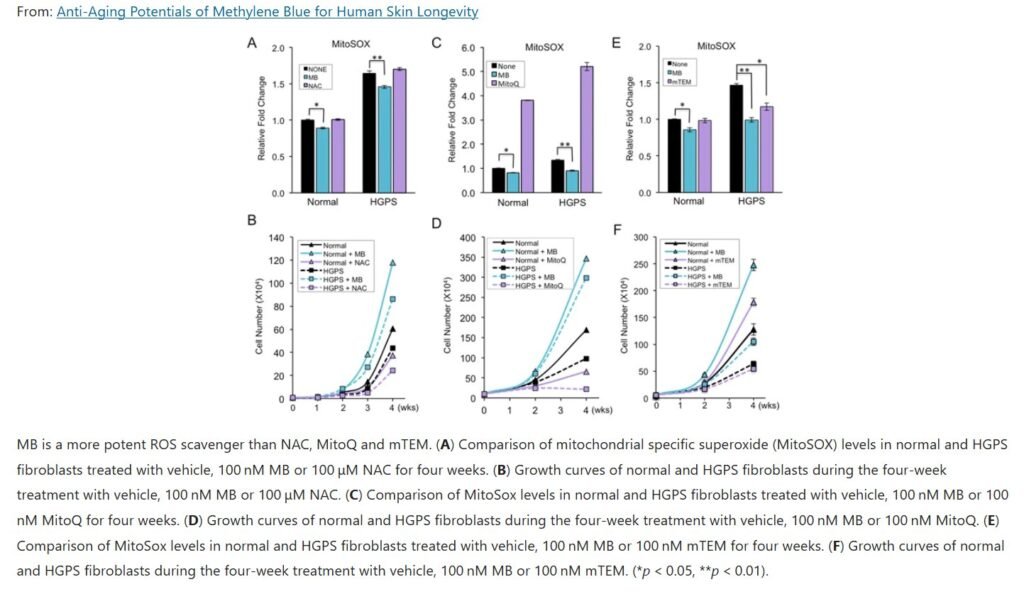
Anti-aging and cellular protection: Methylene Blue has attracted attention as a potential anti-aging compound, thanks to findings that it supports mitochondrial function and reduces cellular wear-and-tear. In lab studies, MB was shown to delay cellular senescence (cell aging) and extend the lifespan of cultured human cells . One landmark 2015 study on cells from children with progeria (a rapid-aging disease) found that low-dose MB could almost completely repair the cells’ defects . Treated progeria cells looked and functioned like normal, and even healthy aging cells showed reversal of some aging markers . “It’s like magic,” the lead researcher remarked . In mice, MB has been reported to increase lifespan (in certain strains) and to preserve memory as they age . These age-defying effects are credited to MB’s lowering of oxidative stress and maintenance of efficient energy metabolism in cells. By acting as a recycling antioxidant (accepting and donating electrons repeatedly) , MB neutralizes free radicals before they can damage mitochondria and other cell components. It also activates the cell’s own antioxidant responses (e.g. upregulating Nrf2, a guardian of cell health) . All this suggests MB might slow aspects of cellular aging and keep tissues (like brain, skin, and heart) functioning better for longer. Indeed, some have proposed MB as a treatment to “expand mitochondrial reserve capacity” in the brain, potentially buffering against age-related decline .
Neurodegenerative diseases (Alzheimer’s, Parkinson’s): Given its neuroprotective and metabolic benefits, Methylene Blue is being intensively studied for neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). MB has multiple actions relevant to these diseases: it can inhibit the abnormal aggregation of the tau protein (a hallmark of Alzheimer’s) and even reduce levels of beta-amyloid plaques in models . It also boosts brain cell metabolism and neurotransmitters like acetylcholine, which tend to be deficient in dementia . Early experiments showed promise – in cellular models, MB prevented the toxic clumping of proteins seen in Alzheimer’s and protected neurons from degeneration . In animal models of Alzheimer’s and Parkinson’s, MB has mitigated neurodegeneration and preserved cognitive and motor function . These benefits led to human trials: a modified form of Methylene Blue (called LMTM or TRx0237) was tested in phase III clinical trials in Alzheimer’s patients. The results were mixed – when added on top of standard Alzheimer’s medications, high-dose LMTM did not significantly slow decline. However, in patients who received it as a monotherapy (without other Alzheimer’s drugs), some positive effects were observed . This suggests MB might work best on its own or in early-stage disease, and researchers are continuing to explore optimal dosing and formulations. In Parkinson’s research, MB has shown neuroprotective effects on dopaminergic neurons, partly by enhancing mitochondrial function and possibly promoting removal of defective mitochondria (autophagy) . Although no large PD trials have reported yet, these findings raise hope that MB or its derivatives could slow the progression of neurodegenerative diseases by propping up the cell’s energy factories and preventing protein misfolding. Notably, a 2008 study even speculated that Methylene Blue (at very low doses) “may be able to slow or even cure Alzheimer’s and Parkinson’s” – a bold claim that fueled much interest . While the word “cure” is likely premature, MB remains a compelling multi-target approach for these currently incurable illnesses.
Athletic performance and energy: A growing number of biohackers and athletes are experimenting with Methylene Blue as a performance enhancer. The logic is straightforward: if MB improves mitochondrial ATP production and oxygen utilization, it could boost muscular endurance and reduce fatigue. Some preliminary reports and animal studies support this idea. By increasing the efficiency of the electron transport chain, MB might help muscle cells extract more energy from each molecule of fuel and oxygen. It also appears to reduce lactic acid buildup (a cause of muscle burn) by improving aerobic metabolism . Coaches and integrative medicine clinics claim that MB can “enhance aerobic capacity, increase endurance, and improve muscle recovery” for athletes . For example, in endurance exercises, MB might delay the point of exhaustion by keeping the mitochondria energized and quenching excess nitric oxide (which, in overabundance during intense exercise, can dampen mitochondrial respiration). Some users report needing less rest and experiencing quicker post-workout recovery when taking micro-doses of MB. However, it’s important to note that formal studies in humans are limited. One older study in dogs found no significant change in oxygen consumption during exercise with MB, suggesting any benefit might be subtle or context-dependent. Still, given its benign profile at low doses, MB is being touted as a “legal mitochondrial enhancer” in sports circles. Athletes seeking an edge – especially in endurance sports – are intrigued by the prospect of cellular-level optimization as opposed to stimulants. More research is underway to quantify MB’s effects on exercise performance, but early indicators show it could help by improving how efficiently muscles use oxygen and generate energy . Even if one isn’t an athlete, this translates to potentially higher daily energy levels and stamina for ordinary people using MB responsibly.
Methylene Blue’s potential benefits span from better memory and mental clarity, anti-aging effects, neuroprotection in diseases like Alzheimer’s/Parkinson’s, to enhanced cellular energy for physical performance. It’s rare for a single compound to impact so many domains – but MB’s unique action on the fundamental process of energy metabolism gives it an unusually broad scope. That said, realizing these benefits in practice requires careful dosing and more clinical research, as we’ll discuss.
Risks and Side Effects
Despite its many upsides, Methylene Blue is not without risks. As a potent bioactive compound (and dye), it must be used judiciously. Common side effects at therapeutic doses include headaches, dizziness, nausea, abdominal pain, and vomiting . These symptoms are generally mild and transient. MB will also turn your urine blue-green (and sometimes feces or sweat a blue tinge) – a harmless but surprising side effect due to the dye passing through . If taken orally, it can temporarily stain the mouth or tongue blue (some modern formulations are put in capsules or lozenges to avoid this). Such cosmetic effects are benign, but they can be inconvenient – for example, clinicians historically knew who took their malaria pill because of the telltale blue urine !
More serious adverse effects tend to emerge with higher doses or in specific at-risk individuals. One major caution is serotonin syndrome: Methylene Blue at high doses can inhibit the enzyme MAO-A, which normally breaks down serotonin. If someone is on antidepressants (SSRIs, SNRIs, MAO inhibitors, etc.), combining them with MB can lead to dangerously high serotonin levels in the brain. The FDA has issued warnings about this – there have been cases where IV Methylene Blue triggered serotonin syndrome (agitation, high fever, rapid heart rate, seizures) in patients on SSRI antidepressants . Thus, MB should be avoided or used with extreme caution in anyone taking serotonergic psychiatric medications . Many doctors recommend stopping SSRIs at least two weeks before elective MB administration (for example, if MB is used during certain surgeries as a dye) to be safe.
Another risk group is people with G6PD deficiency, a genetic enzyme deficiency common in parts of Africa, the Middle East, and Asia. These individuals have red blood cells that are vulnerable to oxidative stress. Methylene Blue can cause oxidative damage to red blood cells in G6PD-deficient people, leading to hemolytic anemia (bursting of red cells) . This is the same reason such patients cannot take drugs like primaquine. While recent studies suggest low doses of MB (as used for malaria) are actually tolerated even in moderate G6PD deficiency , it’s still a contraindication to use MB in known G6PD-deficient patients unless in a life-saving scenario. If MB must be given (e.g. for methemoglobinemia in an emergency), it should be done under close medical supervision.
Methylene Blue is also considered unsafe in pregnancy, as it can cross the placenta and potentially cause fetal harm . Cases of neonatal intestinal atresia (gut issues) have been reported when MB was used in pregnant women for amniotic leak tests. Therefore, pregnant or breastfeeding women are advised to avoid MB. In children, MB is used medically in certain cases (e.g. pediatric methemoglobinemia), but the dose must be carefully adjusted by weight.
It’s critical to understand that dose makes the poison with Methylene Blue. At low doses, MB is an antioxidant and metabolic enhancer; but at high doses, it paradoxically becomes a pro-oxidant and can impair mitochondrial function . There is a U-shaped dose-response curve. Studies have found that low micromolar concentrations improve cell survival and memory in animals, whereas very high concentrations (or overdoses) actually inhibit energy metabolism and increase oxidative stress . In practical terms, more is not better – taking too much MB can backfire, causing cellular oxidation and even methemoglobinemia (the very condition it treats at proper doses!). Indeed, if MB is given in excessive doses intravenously, it can cause methemoglobinemia by overwhelming the red cell reduction systems . Symptoms of MB overdose include chest pain, shortness of breath, a grayish skin discoloration (from methemoglobin), severe nausea, and restlessness. High doses can also precipitate hypotension or arrhythmias.
To maximize benefits and minimize risks, proper dosing is key. Medically, Methylene Blue is typically used in doses around 1–2 mg per kilogram of body weight IV for acute conditions like methemoglobinemia . Orally, clinical doses have ranged from about 50–300 mg per day depending on the condition . Nootropic users, however, often take much smaller “microdoses” – on the order of 5–20 mg total per day – which are well below traditional medical doses. Such low doses (sometimes called “nootropic dosing”) are generally considered safe and unlikely to cause major side effects, while still being sufficient to elevate brain MB levels. For example, in depression trials, just 15 mg per day of MB showed efficacy with minimal side effects . It’s recommended to start at a low dose (e.g. 5 mg) and titrate up only if needed, since individuals can vary in sensitivity. Importantly, any concurrent medications must be reviewed for interactions (especially antidepressants as noted).
Other safety considerations include: ensuring one uses a high-purity, pharmaceutical grade Methylene Blue. Industrial or lab-grade MB (such as aquarium fish tank cleaner) may contain heavy metal impurities like arsenic or mercury. Ingesting such non-pharma grade MB is dangerous. Unfortunately, some unregulated online suppliers sell MB intended for staining or aquariums to consumers. This has led to doctors warning against “drinking fish tank cleaner to stay young,” as some misinformed influencers have promoted . Only medical-grade MB with known purity should ever be taken by humans. Additionally, people on MB therapy are often advised to avoid excessive exposure to strong light. MB is a photosensitizer (it produces singlet oxygen when exposed to bright light), so high doses in combination with intense light (especially UV light) could theoretically cause skin/photosensitivity reactions or eye damage. Though this is mostly a concern in photodynamic therapy contexts, it’s wise to avoid tanning beds or unnecessary UV exposure if taking MB regularly.
Methylene Blue is quite safe at low doses – indeed, it has been used in humans for over 100 years. But it must be respected as a drug. Potential side effects range from benign blue urine and GI upset to more serious issues like serotonin syndrome or anemia in special populations. Sticking to recommended dosages and pharmaceutical-grade sources, and being aware of drug interactions, are crucial for safe use. When used properly, MB’s risk profile is manageable (low-dose MB has even been given chronically for years in some patients for other conditions without major problems). Yet, given the current trend of experimental use, each individual must weigh the unproven benefits against these known risks, ideally with medical guidance.
Current Hype and Popularity
In recent years, Methylene Blue has experienced a resurgence of popularity, fueled by biohackers, longevity enthusiasts, and even some celebrities. It has been touted online as a sort of “miracle nootropic” and anti-aging elixir – a remarkable second act for a 19th-century dye. Why is Methylene Blue trending now? A few converging reasons:
Cutting-edge research meets social media: Exciting research findings over the last 10–15 years (showing MB’s potential to repair cellular aging, enhance memory, etc.) have trickled into the public consciousness. For instance, a 2015 study from the University of Maryland grabbed headlines by demonstrating that MB could reverse cellular aging signs in progeria and normal cells . Around the same time, neuroscience studies were reporting cognitive benefits of MB in animals and humans. These scientific developments primed MB as a “hot new” compound for brain health and longevity – despite it being old, the applications were new. Biohacker communities on Reddit and forums picked up on these papers and began experimenting with MB as a supplement, often reporting increased focus and energy. The fact that MB is inexpensive and unpatented made it readily accessible compared to trendy supplements.
The real explosion in hype came via influencers and social media in the early 2020s. Notably, in 2021–2022, several fitness and wellness influencers on TikTok and Instagram started posting about their personal use of Methylene Blue. They often shared selfies of their tongues stained bright blue (a side effect of some oral MB products) as a badge of biohacker cred. Posts from figures like fitness personality Ben Greenfield (who has ~300k+ followers) extolled MB as a “potent cognitive enhancer” and mitochondrial booster . Greenfield and others claimed that MB “enhances mitochondrial function, provides neuroprotective effects against brain inflammation, increases memory and cognitive function,” and even “enhances the effects of light and oxygen therapies” . Such glowing endorsements, phrased in layman-friendly terms, made MB sound like a must-have brain hack. Similarly, other users on TikTok described it as protecting against neuroinflammation and a “potential therapeutic for Alzheimer’s, Parkinson’s and dementia,” citing its use in research . These claims (some accurate, some a bit exaggerated) were delivered in short, viral videos that reached millions. MB even got tagged as the “smurf drug” on TikTok due to the blue tongue trend.
It also didn’t hurt that taking MB is visually striking – the blue tongue photos became a viral curiosity. This gave Methylene Blue a distinct “wow factor” on social media that most supplements lack. It became a trendy talking point: “Why are people’s tongues blue? What is this methylene blue craze?” Articles in mainstream outlets like the New York Post and The Independent picked up on the phenomenon, noting that some wellness influencers were ingesting aquarium-grade methylene blue (meant for fish tanks) in hopes of anti-aging benefits . Doctors quickly slammed this practice, warning that non-medical grade MB could be dangerous and that these anti-aging claims were not yet proven . The controversy perhaps only fanned the flames – as often happens, being “forbidden” made the trend more intriguing to biohackers.
Another driver of the hype is the general “longevity movement” and endorsement by figures in that space. High-profile biohackers like Dave Asprey and physicians in the functional medicine community began discussing Methylene Blue in podcasts and conferences. They often frame MB as a tool to address “mitochondrial dysfunction,” which is increasingly recognized as a root cause of many chronic diseases and aging. There’s an appealing narrative that by taking a few drops of this blue solution, you’re repairing your mitochondria and potentially extending your healthspan. Methylene Blue dovetailed perfectly with other popular trends – people taking NAD+ boosters, CoQ10, intermittent fasting – all geared toward enhancing cellular energy and aging defenses. It offered a novel mechanism (directly feeding electrons to the ETC) that complemented these other approaches, which made it easy to integrate into the biohacking zeitgeist.
Public figures have played a role too. For example, viral images showed Twitter CEO Jack Dorsey and podcast host Tucker Max trying a product called “Blue Cannatine” (a nootropic troche that includes methylene blue, caffeine, nicotine, and CBD). Their blue-stained mouths on social media sparked curiosity and lent a bit of celebrity endorsement by association. Entrepreneur/author Tim Ferriss also mentioned experimenting with low-dose MB for cognitive benefit in one of his blogs, adding to its credibility among biohackers.
COVID-19 pandemic and wellness trends: During the COVID pandemic, interest in immune and metabolic boosters surged. Some fringe theories even proposed Methylene Blue could treat COVID-19 (due to its antiviral properties in vitro) . A few clinics started offering MB IVs or injections as part of “immune boosting” drips. Although not mainstream, this contributed to discussion around MB in the context of immune health and inflammation. The timing coincided with people being more experimental with supplements at home, which may have helped the MB trend grow.
In summary, Methylene Blue’s current popularity is a product of promising science translated (perhaps a bit too enthusiastically) into social media content. It’s been branded as a cognitive enhancer, anti-aging drug, and athletic aid – essentially a panacea – by various online health movements. The viral blue tongue selfies and endorsements from biohacking influencers gave it a trendy, almost edgy image. As a result, what was once an old malaria drug is now riding the wave as a modern “nootropic cocktail” ingredient and longevity supplement. Sales of pharmaceutical-grade Methylene Blue to consumers (through specialized pharmacies or supplement companies) have reportedly skyrocketed in the past couple of years.
Of course, with hype comes scrutiny. Medical experts stress that many of these uses are not yet FDA-approved or thoroughly researched in humans . They caution that people should not self-medicate with fish-tank cleaner or assume more MB equals more benefit. Nonetheless, the allure of Methylene Blue as a low-cost, scientific yet almost magical-sounding compound has captured the imagination of many looking for an edge in health and performance. It sits at the intersection of DIY biohacking culture and legitimate biomedical research – a rare position that few substances achieve. Whether it lives up to all the hype is still under investigation, but its “cult status” in the wellness world is firmly established for now.
Production and Market Data
Originally produced as a textile dye in Germany (BASF held one of the first patents in 1877 ), Methylene Blue today is manufactured by numerous chemical and pharmaceutical companies worldwide. It is a cheap, off-patent compound, usually synthesized from aniline derivatives. Major producers of Methylene Blue include specialty chemical companies and pharma suppliers. According to industry analyses, some of the largest manufacturers globally are BiTe Chemical, Eastman Chemical, Macsen Laboratories, and Vanshi Chemicals . Big names like Merck (Sigma-Aldrich), Acros, and ThermoFisher also produce MB for laboratory and medical use, and historically BASF was a key producer (given their role in its invention). On the pharmaceutical side, companies like Provepharm (France) have developed high-purity injectable forms. In the U.S., American Regent markets the FDA-approved injectable Methylene Blue (brand name ProvayBlue), which was approved in 2016 . There are also compounding pharmacies that prepare oral MB capsules or troches for prescription use.

When it comes to consumption, Methylene Blue’s market spans multiple sectors: medical, research, and even aquaculture. A 2023 market report estimated the global Methylene Blue market at around USD $7 million, projected to grow modestly . Geographically, Europe is the largest consumer, accounting for about 30% of global MB use, followed by North America at about 20% . Europe’s lead may be due to higher usage in diagnostic medicine and historical use in certain therapies, as well as significant laboratory research consumption in EU countries. North America also has substantial usage, particularly in healthcare (hospitals stocking it as an emergency antidote) and scientific research. Interestingly, regions with ongoing malaria control programs (parts of Africa and Asia) use MB in combination therapies, but the volumes there are still relatively small compared to mainstream pharmaceuticals like chloroquine or artemisinin.
In terms of applications, the pharmaceutical/medical sector is the largest consumer of Methylene Blue (about 85% of the MB market by volume is of high purity 98.5–99% grade MB used for medicine) . This includes its use in hospitals for methemoglobinemia, in surgical settings as a dye (e.g., to visually trace fistulas or sentinel lymph nodes), and in diagnostic products like the new Lumeblue colonoscopy tablets. The next biggest application is biological staining in laboratories . MB is widely used in microscopy to stain tissue samples and bacteria (e.g., Wright’s stain for blood smears contains MB, and many school biology labs use MB to stain cell nuclei). It’s a staple dye in microbiology for Gram stain procedures and in histology, so academic and industrial labs are steady consumers. Another notable chunk is aquaculture and pet trade: MB is sold as an antifungal and antiparasitic treatment for fish tanks (if you’ve treated aquarium fish for ick, you might have used MB). It’s also used in some wastewater treatment processes. However, these uses typically involve lower grades of MB (industrial grade) and account for a smaller portion of revenue.
The renewed interest in MB as a supplement has created a niche market of direct-to-consumer sales. A few companies now sell food-grade or pharma-grade Methylene Blue specifically marketed for nootropic or wellness purposes. While hard numbers are not published, online retailers have reported increasing demand. That said, this “biohacker” segment is still tiny relative to the established medical/lab market.
One can gauge MB’s global reach by the fact that it is included on the WHO Essential Medicines list , meaning most countries keep it in stock for critical uses. Countries with large populations and robust healthcare/research systems (USA, China, India, Germany, UK, etc.) inevitably consume a lot of MB. For instance, India and China produce and use significant quantities for both medical and industrial purposes – India’s pharmaceutical industry uses MB in some UTI combo pills and as a process chemical, while China recently approved Lumeblue for colonoscopy which will increase medical MB usage . Africa’s consumption had been low after WWII (when MB use for malaria waned in favor of chloroquine), but in the past decade there’s been a push to reintroduce MB for malaria in combination therapies, funded by international donors . This could raise consumption in sub-Saharan Africa if adopted widely.
In the chemical supply market, MB is so common that many suppliers treat it almost as a commodity chemical (it’s even sold on Amazon for lab use). The prices are modest – for example, in the U.S., a 10 mL vial of 0.5% injectable MB (ProvayBlue) costs on the order of $50–100, while a bottle of 25g lab-grade powder may cost ~$20–30. The relatively low cost and ease of production (it’s been made for 145+ years) mean there’s less incentive for huge pharma investment, but steady demand keeps a number of manufacturers in business.
In summary, Methylene Blue production is globally distributed with key players in North America, Europe, and Asia. Europe currently leads in usage (likely due to broad medical and research use), with North America next . The main uses driving the market are medical (therapeutic and diagnostic) and scientific staining, with a small yet growing segment of health supplement use. Given its versatile roles – from saving lives in methemoglobinemia to tinting microscope slides – Methylene Blue maintains a firm, if unglamorous, presence in the world market. The recent buzz may expand its consumer market a bit, but its core remains in healthcare and science.
Past and Ongoing Research
Methylene Blue has one of the most storied histories in pharmacology – its research journey spans over a century, with many twists and turns. Here, we outline some key historical studies and the latest research directions:
Early research (1880s–1920s): After Ehrlich’s discovery of its antimalarial effect in 1891 , MB became a prototype for the emerging field of “chemotherapy” (using chemicals to treat disease). By the late 1890s, researchers were testing MB on everything from tuberculosis (Robert Koch used it as a TB stain and noted some microbe-killing effects) to mental illness. One of the first published psychiatric trials was in 1899, where MB was given to patients with psychosis; some improvements were noted, making it arguably the first antipsychotic drug used . Though results were inconsistent, these experiments suggested MB’s neurological effects (decades before dopamine was discovered!). In 1920, scientist Matilda Brooks found that MB could act as an antidote to cyanide and carbon monoxide poisoning in mice . Her 1933 paper showed high doses of MB helped rescue asphyxiated animals , opening up a new antidotal use. Also in the early 20th century, MB’s ability to convert methemoglobin back to normal hemoglobin was elucidated – this became a standard treatment after methylene blue saved soldiers from chemical-induced methemoglobinemia in WWI. By the 1940s, MB was well established for methemoglobinemia and had seen intermittent use for malaria (until chloroquine took over).
Mid-century research (1930s–1960s): With the rise of antibiotics and new antipsychotics, interest in MB waned somewhat. Yet, some important findings emerged. In the 1930s it was noted that MB is a monoamine oxidase inhibitor (MAOI) – making it one of the earliest MAOIs known . This helped explain its antidepressant effects observed in some patients. In the 1960s, scientists Lindahl and O’berg (1961) and others in 1960s–70s worked out MB’s mitochondrial role, noting it could carry electrons and reduce oxygen consumption in certain conditions . This was largely academic interest at the time. Also, pharmacologists studied MB’s enzyme interactions: it inhibits guanylate cyclase and nitric oxide synthase, which suggested it could modulate blood vessel tone (indeed MB was later used in vasoplegic shock to raise blood pressure by blocking nitric oxide signaling ). These mechanistic studies kept MB in textbooks as a pharmacological tool.
Clinically, in 1975 a South African psychiatrist, Dr. Rankin, reported success using low-dose MB to treat bipolar disorder (as a prophylactic to prevent depressive episodes). In the 1980s, Narsapur & Naylor (1983) conducted a controlled trial in severe depression, finding that MB (15 mg thrice daily) significantly helped depression scores vs. placebo . This was a landmark showing a psychiatric use. Another curious mid-century use: MB was used as a urinary antiseptic in combo with phenyl salicylate and benzoic acid (the formulation “Urolene Blue”) especially in the 1940s-50s for UTI symptoms; though not truly effective as an antibiotic, it did provide symptom relief and turned the urine blue which assured patients something was happening.
Modern research renaissance (2000s–present): Starting around the early 2000s, there has been a renaissance in Methylene Blue research, especially in neurology and aging. Pioneering work by Gonzalez-Lima and colleagues around 2004 demonstrated that low-dose MB enhances memory retention in rats, likely by increasing brain cytochrome oxidase activity (a marker of mitochondrial function) . This sparked new interest in MB as a cognitive enhancer. In 2008, an influential paper by Atamna et al. showed that MB can extend the lifespan of normal human fibroblast cells by ~20% and improve mitochondrial biochemical pathways . That same year, Atamna and co-authors wrote a provocative article calling MB a possible “cellular rejuvenation” agent, which, along with a press release, led to the ScienceDaily piece titled “Century-Old Drug Reverses Alzheimer’s and Parkinson’s in the Lab” . Although that title was optimistic, the data did show MB improving mitochondrial function in a mouse Alzheimer model and preserving cognitive function . Around 2009, a small trial in claustrophobia found that a single dose of MB before exposure therapy enhanced extinction of fear (basically, people got over their phobia faster) – highlighting a memory-extinction benefit relevant to PTSD therapy.
With these encouraging signals, clinical trials accelerated. TauRx Therapeutics sponsored large Phase II and III trials of MB (methylthioninium) in Alzheimer’s disease between 2008–2016. The Phase II (2008) had reported slower decline in MB-treated patients, raising huge hopes. Phase III results in 2016, however, were largely negative except for a subset, as mentioned earlier . This was a setback, but research continued to dissect the results and optimize protocols (TauRx is ongoing with another trial as of 2022, using MB as monotherapy in early AD). In parallel, other studies investigated MB in Parkinson’s models, finding neuroprotective effects (for example, MB protected dopamine neurons from MPTP toxin in mice by inducing protective factors like BDNF) . MB was also tested in stroke models – a 2018 study found MB given after a stroke in rats reduced brain damage by sustaining mitochondrial function in the penumbra (area around the infarct). Likewise, research in traumatic brain injury and spinal cord injury models showed improved outcomes with MB treatment .
Another fascinating line of inquiry is combining MB with light therapy. Since MB can absorb light (especially red/near-infrared) and convert it to a form of energy, some researchers are pairing MB with low-level light (photobiomodulation) to treat neurological conditions. Early cell studies indicate the combo can synergistically boost mitochondrial function and possibly spur neuron repair . Clinical trials are being planned to see if MB + red light can help Alzheimer’s or chronic brain injury patients.
Beyond neurology, MB is being revisited for infections and other illnesses. Its antimalarial use is being revived: trials in Africa (2018) showed that adding low-dose MB to standard malaria treatment cleared parasites faster and also had the benefit of making patients’ blood non-infectious to mosquitoes sooner . The main drawback was the blue urine causing compliance issues, but research is ongoing into MB as part of artemisinin combination therapies for malaria given rising drug resistance. MB’s antiviral properties have also come into focus – it can inactivate viruses when combined with light (it’s used to sterilize blood transfusions for viruses like HIV in some countries). During the COVID-19 pandemic, there were exploratory studies of MB (with and without light) in treating COVID patients, given its antiviral and anti-inflammatory actions . Some small trials (e.g. in Iran) claimed faster recovery in MB-treated COVID patients, but these are not yet mainstream and larger studies are needed. Nevertheless, it underscores MB’s broad biomedical interest.
The research on Methylene Blue is very active. Key areas of ongoing research include:
• Neurodegenerative diseases: Optimizing MB derivatives or protocols for Alzheimer’s and Parkinson’s. (E.g., testing MB in earlier stages of Alzheimer’s, or combining with other therapies. Investigating MB’s effect on tau protein and amyloid in human patients via imaging trials.)
• Neuroprotection and cognitive enhancement: Trials in mild cognitive impairment, post-stroke recovery, PTSD (using MB to enhance psychotherapy), and age-related cognitive decline are either ongoing or in development. Researchers are also examining if MB can stimulate neurogenesis (new neuron growth) – some rodent data suggests it might .
• Psychiatric disorders: There’s renewed interest in MB for mood disorders. A recent trial (2017) in bipolar depression found that adding MB (methylene blue 15 mg daily) to lithium improved depressive symptoms more than lithium alone. Further studies are exploring MB as an adjunct in difficult-to-treat unipolar depression and anxiety disorders (leveraging its neurotransmitter modulation).
• Metabolic and mitochondrial diseases: Given MB’s mitochondrial boost, researchers are testing it in conditions like chronic fatigue syndrome, fibromyalgia, and mitochondrial genetic disorders. While mostly anecdotal so far, there is a logic that MB could ameliorate fatigue and exercise intolerance in those with mitochondrial dysfunction.
• Anti-aging in humans: No official anti-aging trial in humans has been completed yet, but there is talk of designing one (perhaps measuring biomarkers of aging in people taking MB vs placebo over a year or more). Meanwhile, studies in model organisms (like worms, flies, mice) continue to assess MB’s effect on lifespan and healthspan. Notably, a study on middle-aged mice (2016) found MB extended their remaining lifespan and improved memory tests, but more studies are needed for confirmation.
• Alternate formulations: Ongoing R&D aims to create better MB formulations – for example, leucomethylene blue (the reduced form) which might cross the blood-brain barrier more efficiently, or nanoparticle delivery of MB to specific organs. Lumeblue (the colonoscopy pill) is an example of formulation innovation for diagnostics. There is also interest in topical MB for skin aging; a 2017 lab study showed that MB outperformed vitamin C and retinol in a skin culture model of wrinkle formation, which has led some cosmetics researchers to look at MB-containing skin creams for anti-wrinkle effects. A small cosmetic trial is underway to see if a low concentration MB cream can improve skin elasticity or fine lines in humans.
Overall, after a long period of relative obscurity, Methylene Blue is once again a hot topic in research. It’s somewhat rare for a drug of this age to get new indications, but MB’s multifaceted actions have opened numerous investigative paths. Importantly, many of MB’s potential uses are being evaluated in clinical trials right now. The outcomes of these trials in the next few years will determine if Methylene Blue transitions from an off-label experimental therapy to a widely approved treatment for conditions like Alzheimer’s or persistent Lyme disease (another niche where MB is being experimented with). The scientific community is cautiously optimistic – MB has already surprised us several times in history, and it may have more to offer as research continues.
Regulatory Landscape
The regulatory status of Methylene Blue varies by country, but generally it is treated as a prescription pharmaceutical rather than a dietary supplement. Here we compare how it’s handled in the U.S. and Europe, along with approved uses:
United States (FDA regulations): In the U.S., Methylene Blue is an FDA-approved drug for a few specific indications. The FDA has approved MB (as ProvayBlue injection) for the treatment of acquired methemoglobinemia . This approval came in 2016, formalizing a use that had been standard in emergency medicine for decades. ProvayBlue (methylene blue 0.5% in 10 mL vials) is prescription-only. The FDA label for ProvayBlue includes a boxed warning about serotonin syndrome, reflecting the risk when combined with serotonergic psychiatric medications . Another FDA-approved product is Urolene Blue, a legacy oral formulation for urinary tract infections; however, this indication is obsolete and such products are no longer commonly marketed. Currently, there are no FDA-approved indications for Methylene Blue in cognitive impairment, Alzheimer’s, etc. Any such use is off-label. MB is also used off-label in ifosfamide-induced encephalopathy and in refractory vasodilatory shock, but these are not official FDA-approved uses.
Importantly, the FDA does not allow Methylene Blue to be sold as a dietary supplement. It is considered a drug. In fact, in recent years as MB supplements popped up online, the FDA and FTC have issued warnings to certain vendors. For example, a June 2020 FTC warning letter targeted a company selling “Methylene Blue bottles” with unproven COVID-19 cure claims . The FDA has also cautioned consumers that products containing MB for cognitive enhancement are unapproved new drugs. That said, it is legal for compounding pharmacies to dispense MB with a doctor’s prescription (and many do, often prescribing low-dose MB capsules to patients under a doctor’s care for off-label uses).
In the U.S. you cannot buy Methylene Blue over-the-counter for ingestion; it must be prescribed or it’s sold as a chemical for lab use (not labeled for human consumption). The FDA’s stance is cautious, given the lack of large trials for nootropic or anti-aging use. Any future claims for those uses would require substantial evidence and new drug approvals.
European Union (EMA regulations): In the EU, Methylene Blue (often referred to as methylthioninium chloride) is likewise regulated as a medicine. It has been used in Europe for methemoglobinemia for decades (often under names like “Methylthioninium Chloride PROVEBLUE”), and the European Medicines Agency (EMA) has it authorized for that indication. Recently, Europe actually moved ahead of the U.S. in approving a new use: In 2020, the European Commission approved Lumeblue – an oral methylene blue tablet – for use in colonoscopy to enhance visualization of polyps . Lumeblue (developed by Cosmo Pharmaceuticals) delivers MB throughout the colon, staining lesions blue to help endoscopists detect more precancerous polyps. This was based on successful phase III trials showing improved adenoma detection rates . So in the EU, methylene blue now has an official diagnostic indication in addition to therapeutic ones. (By contrast, the FDA has not yet approved Lumeblue – they issued a Complete Response Letter asking for a second trial , so it’s not yet available in the U.S. for colonoscopy use.)
EU countries also historically used MB in combination UTI drugs (similar to the U.S.), but these have largely fallen out of favor. One notable difference: In some European countries, low-dose oral MB has been used in psychiatry research – for instance, in the UK and Canada (not EU, but related practices) some psychiatrists legally prescribed MB for bipolar disorder adjunct therapy in the 2010s. However, there’s no EMA-approved psychiatric indication yet; those uses remain off-label and experimental.
From a regulatory perspective, the EU classifies MB as a prescription medication. It would be against regulations to market it as a general consumer supplement claiming cognitive or anti-aging benefits. If companies tried, they would run into the EU’s medical claims laws. So far, we haven’t seen a large supplement trend in Europe as much as in the U.S., possibly because regulations and culture are stricter about self-medication. However, Europeans interested in MB can sometimes obtain it through compounding pharmacies or chemical suppliers, similar to the U.S.
Other countries: Methylene Blue is in the pharmacopeias of most countries. For example, in India and many Asian countries, MB is available in hospitals and sometimes in pharmacies (often for UTIs or as a lab reagent). Some countries allow a form of MB to be sold OTC for fish tank or water purification uses, which some individuals misuse for self-dosing. Regulators universally advise against that.
Differing approaches (US vs EU): Both the FDA and EMA are cautious, but the EMA has been a bit more forward-thinking in approving new uses like Lumeblue. The FDA tends to want more robust evidence (as seen by the delayed approval for the colonoscopy use). When it comes to novel claims like nootropic or anti-Alzheimer’s effects, both jurisdictions would require significant clinical trial evidence to approve MB for those indications. TauRx (the company behind the Alzheimer’s trials) has sought approvals – so far none of the major agencies (FDA, EMA, MHRA) have approved MB for dementia, given the ambiguous trial results. If future trials are positive, we could see an application for an Alzheimer’s indication.
Another aspect is safety communications: The FDA has actively communicated about MB’s risks (issuing Drug Safety Communications on the serotonin syndrome issue in 2011) . The EMA similarly has warnings in place. Both require labeling that contraindicates MB with SSRIs. The U.S. label also contraindicates it in G6PD deficiency and advises caution in pregnancy. These contraindications would be similar under EMA rules.
Access and scheduling: Methylene Blue is generally not a controlled substance (it’s not scheduled as it has no abuse potential). It’s simply a prescription drug. In the U.S., that means it’s relatively easy for a doctor to prescribe off-label if they see fit. The same in Europe – a doctor could prescribe it off-label (say, for an Alzheimer’s patient in a compassionate use scenario). However, insurance coverage would typically only apply for approved indications (like methemoglobinemia). For off-label cognitive or experimental use, patients likely pay out of pocket. Because MB is cheap, this is not a huge barrier – a month’s supply at low dose might cost $20–50 via compounding pharmacy.
Supplement market enforcement: In the U.S., the presence of MB products on Amazon and nootropic websites has prompted some enforcement. Amazon actually disallowed sales of ingestible MB for a while due to FDA pressure. Now one usually finds MB on Amazon marked “for laboratory use only” with no dosage info. The FTC also has cracked down on any outrageous claims (especially around COVID cure-alls) . Overall, regulatory bodies are trying to strike a balance: acknowledging MB’s legitimate medical uses while preventing unsanctioned, potentially unsafe consumer use.
In conclusion, regulators treat Methylene Blue as a drug, not a vitamin. The U.S. FDA permits its medical use for blood disorders and as a dye, but not as a general wellness supplement. The EU EMA similarly restricts it to medical contexts, even as it embraces new diagnostic uses. Both require prescriptions for therapeutic use. Any future expansion of approved uses (such as cognitive impairment or neurodegenerative disease) will depend on successful clinical trial outcomes and subsequent regulatory review. Until then, those experimenting with MB for off-label reasons are operating in a gray zone – legally they should have a doctor’s prescription, and regulators advise caution. The situation is reminiscent of other repurposed drugs (like low-dose naltrexone or ketamine) where enthusiasm runs ahead of official approvals.
Comparison with Similar Compounds
Methylene Blue occupies a unique niche, but it does share some effects with other mitochondrial enhancers and nootropics. Here we compare MB to a few categories of similar compounds, examining relative advantages and drawbacks:
vs. Coenzyme Q10 and other ETC boosters: Coenzyme Q10 (ubiquinone) is a well-known supplement that also targets mitochondria. CoQ10 is actually a natural electron carrier in the ETC (shuttling electrons from Complex I/II to Complex III). Taking CoQ10 can support mitochondrial function, especially in CoQ10-deficient states (like statin medication users or certain mitochondrial disorders). However, Methylene Blue is mechanistically distinct from CoQ10. MB can bypass damaged complexes and directly donate electrons to cytochrome c , whereas CoQ10 still relies on the proper function of Complex I and III to do its job. If Complex I is impaired, just adding more CoQ10 won’t fix the bottleneck, but MB potentially could by rerouting electrons. Additionally, MB has pharmacological effects (MAOI, antioxidant, neurochemical actions) beyond just the ETC, while CoQ10’s main role is as an antioxidant and ETC cofactor. One might say MB is a more direct “electricity donor” to the system. Indeed, some enthusiasts claim “Methylene Blue is 1,000 times more effective than CoQ10” for boosting mitochondrial function – an exaggerated claim, but pointing to MB’s potency. CoQ10 is extremely safe (virtually no side effects even at high doses), whereas MB has the aforementioned risks at higher doses. So CoQ10 may be preferred for general mitochondrial support if one wants a gentle, risk-free supplement. MB could be viewed as a stronger, more interventionist approach – potentially more effective in challenging scenarios, but with more considerations. Interestingly, some anecdotal reports suggest taking CoQ10 and MB together might be counterproductive – possibly because they could in part cancel out each other’s redox cycling (there’s speculation that CoQ10 keeps MB in an oxidized state, reducing its efficacy ). This isn’t well-studied, but it highlights that combining mitochondrial therapies can be complex.
vs. NAD⁺ boosters (e.g. NR/NMN): Nicotinamide adenine dinucleotide (NAD⁺) is the essential electron donor to Complex I. Supplements like nicotinamide riboside (NR) or NMN aim to raise cellular NAD⁺ levels, thereby improving mitochondrial function and activating sirtuins (anti-aging enzymes). These have become popular as longevity supplements. Compared to MB, NAD⁺ boosters work upstream – they ensure the cell has enough “fuel” (NADH) to feed electrons into the ETC. But again, if the ETC is running poorly, simply adding more NAD⁺ might not translate to more ATP. MB, on the other hand, can help utilize whatever NADH is available by keeping the ETC moving or providing an alternate route. NAD⁺ boosters have minimal side effects (some people get mild flushing or nausea). They also have broader systemic effects on metabolism and gene expression via sirtuins. MB doesn’t boost NAD⁺ or sirtuins; it works more directly on the ETC and certain enzymes. One could see NAD boosters and MB as potentially complementary – one increases input (NADH), the other ensures throughput. There’s no direct comparative study, but anecdotally MB’s subjective effects (alertness, mood) are noticed more immediately by users than those of NR or NMN, which may be subtler and long-term.
vs. Antioxidants (vitamin C, alpha-lipoic acid, etc.): Antioxidant supplements help neutralize ROS and support mitochondria indirectly. For example, alpha-lipoic acid can boost mitochondrial enzymes and also regenerate other antioxidants. Methylene Blue, at low doses, also reduces oxidative stress but through a different mechanism – by preventing ROS formation at the source and by its own redox cycling acting as an intracellular antioxidant . MB can additionally trigger the Nrf2 antioxidant response in cells . Compared to standard antioxidants like vitamin C or E, MB might be more potent in the mitochondria because it concentrates there and actively participates in electron flow. A lab study actually found MB was more effective than vitamin C or E in protecting mitochondria from certain stresses . However, antioxidants are very safe and widely used, whereas MB requires more caution. Another antioxidant strategy is mitochondria-targeted antioxidants like MitoQ or SkQ1, which are chemically designed to accumulate in mitochondria (similar idea to MB’s accumulation). Those specifically scavenge ROS in mitochondria. MB’s advantage over them is that it not only scavenges ROS but also improves energy production. But targeted antioxidants might avoid the systemic pharmacologic effects MB has (like MAOI), so they won’t risk serotonin syndrome or other MB-specific issues.
vs. Stimulant nootropics (e.g. caffeine, modafinil, Adderall): Many people seeking cognitive or energy enhancement turn to stimulants. These work very differently – they increase neurotransmitter release or prevent sleepiness, giving a temporary mental boost. For instance, modafinil promotes wakefulness and can enhance focus, and Adderall (amphetamine) increases dopamine/norepinephrine, improving alertness and executive function. Methylene Blue is not a stimulant; it will not directly force the brain into overdrive in the way caffeine or amphetamines do. Instead, MB’s cognitive benefits come from making brain cells run more efficiently (and perhaps increasing neurotransmitters mildly via MAO inhibition ). MB’s effects are subtler and take a bit of time (hours to days) to manifest, whereas a cup of coffee or amphetamine pill will be felt in minutes. The flip side is MB is neuroprotective, whereas stimulants can be neurotoxic if abused. MB might improve cellular health long-term; stimulants do not. For someone looking for immediate intense focus, MB won’t replace Adderall. But MB could enhance baseline cognitive function and brain resilience, which might in turn reduce the need for high stimulant doses.
vs. Other nootropics (racetams, etc.): Nootropic compounds like piracetam, noopept, or bacopa work through various mechanisms – enhancing synaptic plasticity, cholinergic function, or reducing anxiety – but they don’t directly target mitochondria. Piracetam, for example, is thought to improve neuronal membrane fluidity and has minor effects on glucose utilization, but nothing as direct on energy metabolism as MB. One could theoretically stack MB with such nootropics for combined effects. MB’s advantage is that it tackles the fundamental energy supply to the brain – something most nootropics don’t do. Its disadvantage might be that if energy isn’t the limiting factor for someone’s cognition, MB alone might not feel like a cognitive “boost” in the way racetams (which modulate neurotransmission) might. Also, racetams have virtually no known serious side effects and a long track record of use; MB, while generally safe at low dose, still has that serotonin and urine discoloration caveat.
vs. Neuroprotective peptides/other experimental agents: There are other experimental compounds aimed at brain health, like SS-31 (elamipretide) – a mitochondria-targeted peptide that improves ETC efficiency and reduces ROS, used in trials for heart failure and mitochondrial diseases. SS-31, given by injection, is somewhat analogous to MB in end effect, but works by binding to cardiolipin in the inner mitochondrial membrane to stabilize it. Elamipretide has shown promise in improving energetic and exercise capacity in mitochondrial myopathy patients. Compared to MB, it’s more specific (no MAO or other effects) but requires injection and is not as far along for general use. As another example, Creatine is a supplement that improves quick ATP recycling in muscles and brain by buffering phosphate energy; it’s often taken to improve power output and cognitive fatigue. Creatine complements MB: creatine ensures a reserve of ATP is available, MB helps the mitochondria regenerate ATP faster. Both can be part of a mitochondria-supportive regimen (and indeed some neurohackers use both).
Advantages vs Disadvantages: Summarizing, Methylene Blue’s advantages are its multimodal action – it not only supports electron transport (like CoQ10) but also acts as an antioxidant, enhances cerebral blood flow by NO scavenging, increases key neurotransmitters by MAOI action, and can inhibit toxic protein aggregations . Few other compounds check all those boxes in one. This Swiss-army-knife quality means MB can address complex conditions (like Alzheimer’s) on multiple fronts at once . Another advantage is its extensive history of use – we have over a century of data on its safety in various contexts, whereas many new nootropics have unknown long-term safety.
On the disadvantage side, MB’s very multiplicity of effects means it can have interactions and side effects that simpler supplements don’t. For instance, neither CoQ10 nor piracetam will ever give you serotonin syndrome – but MB could if combined incorrectly . Many mitochondrial nutrients (CoQ10, carnitine, NR) are basically nurturing what the body already does, whereas MB intervenes in a more pharmacologic way. Additionally, MB’s vivid color and staining properties create practical issues (blue urine, etc.) that alternatives don’t have.
Another consideration: MB in high doses can actually impair complex IV if over-reduced, whereas something like CoQ10 doesn’t have a known overdose toxicity in the ETC. This means MB has a narrower optimal dosage range – it behaves almost like a hormetic drug (beneficial at low dose, harmful at high dose) , whereas most nutritional mitochondrial enhancers just plateau out without inversion of effect.
One might ask: why not just use oxygen therapies or exercise to boost mitochondria instead of MB? Certainly, regular exercise is one of the best ways to improve mitochondrial function in the long run (it induces mitochondria to grow and become more efficient). MB is more of a targeted acute aid or for when there’s a pathological block in the chain. It doesn’t replace the need for healthy lifestyle (in fact, combining exercise with MB might yield interesting synergistic studies in the future).
While there are many compounds aimed at improving cellular energy and brain function, Methylene Blue stands out for its direct electron-donor capability and broad biological targets . It could be viewed as both a metabolic enhancer and a drug with specific pharmacological actions. Compared to simpler supplements like CoQ10 or NAD boosters, MB may produce more noticeable benefits in certain scenarios (e.g., cognitive tasks, neuroprotection) but comes with more caveats. In the landscape of nootropics, MB is somewhat unique – rather than tweaking neurotransmitters or just providing antioxidant cover, it literally helps the engines run in neurons. This unique modus operandi can complement other approaches: for example, one might use MB alongside cholinergic nootropics (addressing energy + neurotransmitter) for a one-two punch. However, careful consideration of interactions is advised.
Ultimately, the “best” choice depends on the use-case: for a healthy individual looking for a mild boost, established supplements like CoQ10 or caffeine might be sufficient. For someone with a specific mitochondrial issue or wanting to explore cutting-edge biohacks, Methylene Blue offers a novel mechanism that few others do. As research evolves, we may better define where MB fits in the toolbox relative to its peers – possibly as a cornerstone of mitochondrial medicine, used in synergy with diet, exercise, and other compounds to optimize cellular energy and health.
References: Methylene Blue has journeyed from dye to drug, with key historical notes and modern studies illuminating its mechanisms and potential applications in neurology and aging . Its safety profile and interactions are well-documented , guiding current best practices for use. As ongoing trials and biohacker experiments continue, our understanding of this “magic blue” compound will no doubt deepen, potentially consolidating its place in both medicine and personal health optimization.